Improving Analytical Laboratory Management with Real-time Visibility into Test Method QA/QC
Laboratory managers are under increasing pressure to prove that their analytical results are accurate and reliable to their colleagues using those results to make decisions, control processes, and determine the fate of final product. Real-time visibility into lab method QA/QC will meet this need while providing a tool for better laboratory management.
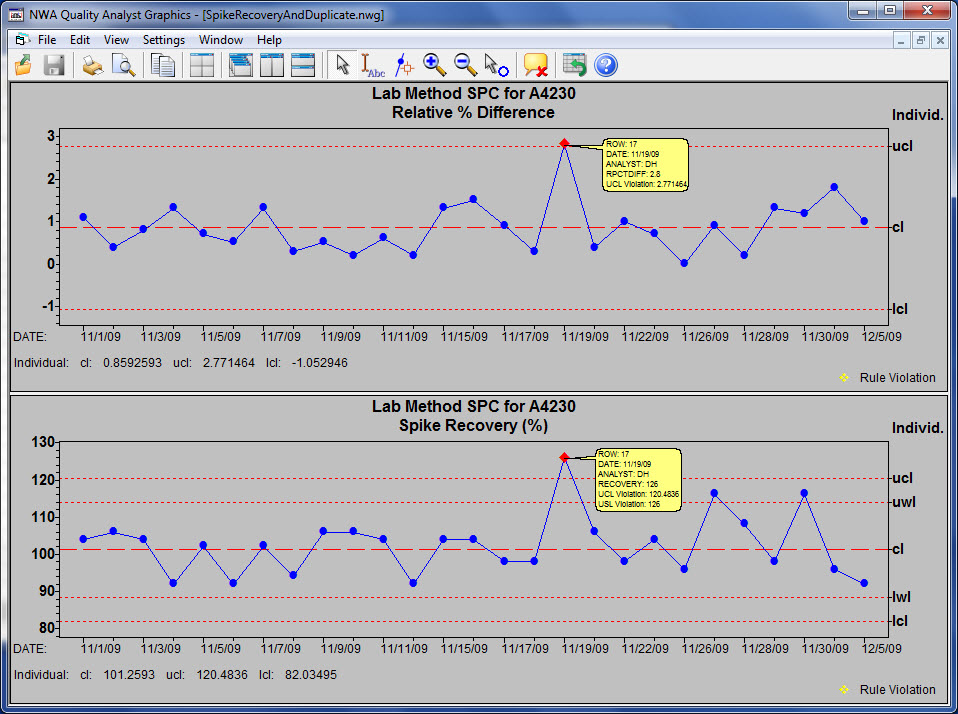 Northwest Analytics has long been the dominant provider of SPC capabilities to analytical laboratories. This is in large part due to NWA Quality Analyst's® range of statistical functions and ease-of-use, as well as our partnerships with the major LIMS vendors. Most of our laboratory customers use NWA Quality Analyst to perform routine lab method QA/QC to meet a wide range of government and industry standards (EPA, FDA, ASTM, NIST, AOAC, etc.).
Northwest Analytics has long been the dominant provider of SPC capabilities to analytical laboratories. This is in large part due to NWA Quality Analyst's® range of statistical functions and ease-of-use, as well as our partnerships with the major LIMS vendors. Most of our laboratory customers use NWA Quality Analyst to perform routine lab method QA/QC to meet a wide range of government and industry standards (EPA, FDA, ASTM, NIST, AOAC, etc.).
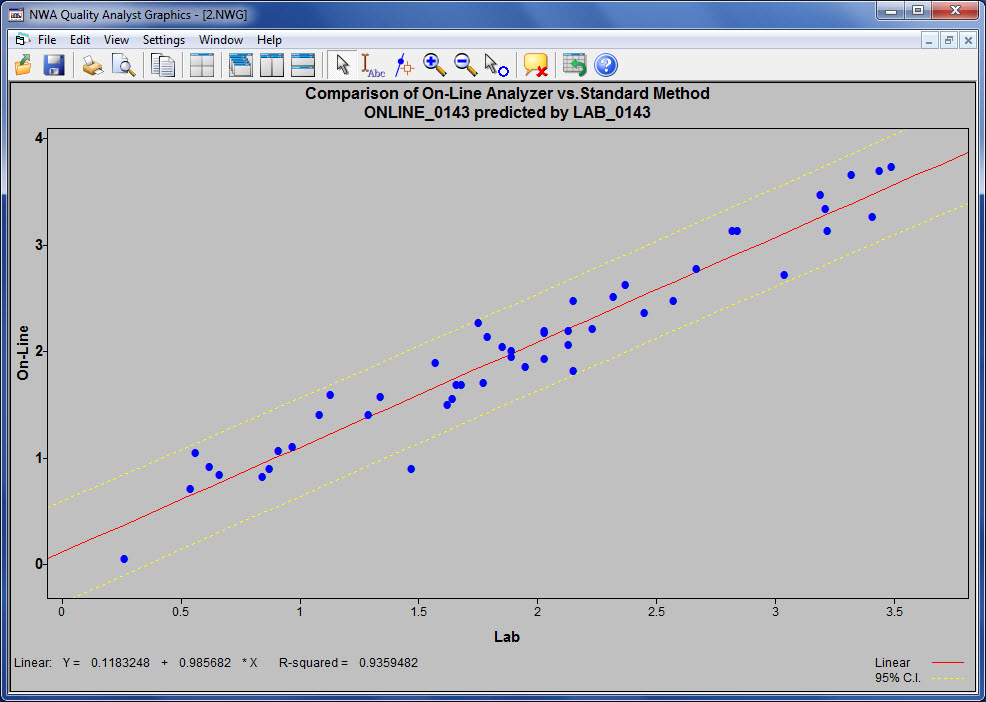
This use of NWA Quality Analyst has met the needs of analytical laboratories for decades, but things are changing. Analytical laboratories supporting refining and chemical process operations are under increasing pressure to verify method performance, particularly when results are used to classify products or validate on-line analyzer performance. Showing compliance with standards such as ASTM 6299, 3764, 6706 and the new EPA 40CFR80 on a continuous basis has become essential.
To meet this need, many of Northwest Analytics' laboratory customers are turning to NWA Focus EMI® to provide real-time visibility into laboratory method QA/QC. NWA Focus EMI uses NWA Quality Analyst data sets and database connections to deliver real-time analytics dashboards and notification services for monitoring process performance and product quality.
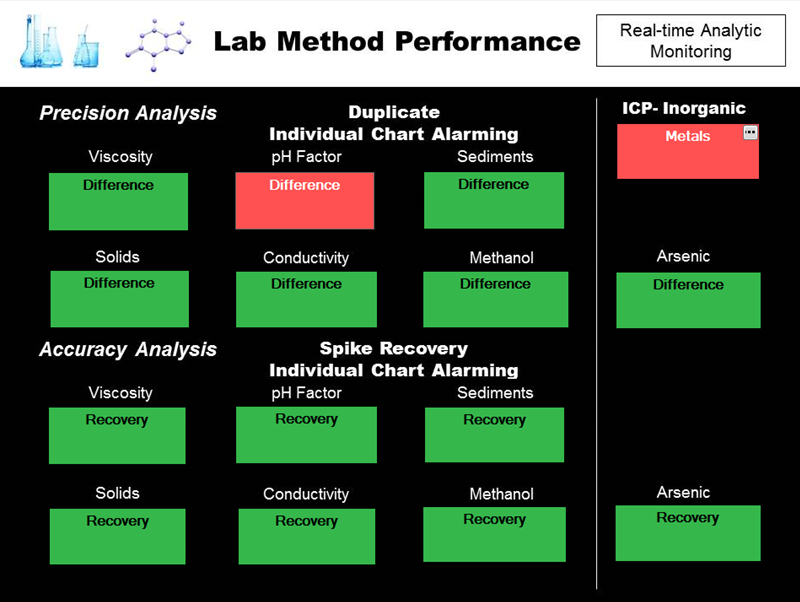 Real-time monitoring and immediate visibility into laboratory test method performance helps build confidence and speed up decision-making based on laboratory results. While this is important for all test results, analyses that result in significant cost savings or losses have much more visibility within the corporation (e.g., final lot clearance, raw material validation, and in-process testing that drives adjustments to batches or process units).
Real-time monitoring and immediate visibility into laboratory test method performance helps build confidence and speed up decision-making based on laboratory results. While this is important for all test results, analyses that result in significant cost savings or losses have much more visibility within the corporation (e.g., final lot clearance, raw material validation, and in-process testing that drives adjustments to batches or process units).
Ensuring – and proving – accuracy is critical to the working relationship between the laboratory and those that use its test results. In one example, constantly monitoring the performance of on-line analyzers and ensuring that they match the laboratory's results from the standard test method has reduced the number of batch adjustments and the amount of down-graded final product, resulting in significant (and highly visible) cost savings.
Contact an account manager today at 503-224-7727 or sales@nwasoft.com to learn more about how analytical laboratories are using real-time visibility of routine lab method QA/QC to build confidence and speed decision-making based on laboratory results.

