Life Sciences
 Life Sciences and Pharmaceutical Industries Solutions
Life Sciences and Pharmaceutical Industries Solutions
Increase production yield…reduce defective product…improve FDA compliance. For more than twenty five years, NWA has delivered the manufacturing intelligence and analytics that enables leading pharmaceutical and life-sciences companies to move from reactive problem solving to proactive process management.
 Given the different process management environments ranging from initial synthesis to packaging, pharmaceutical and life sciences plants face major challenges to understand the total process. NWA solutions help them to collect data at each step of the production process, analyze it, and then make it visible for good decision support. With high ease-of-use and configurable-off-the-shelf approach, NWA solutions have a minimal impact on IT and a fast time-to-ROI.
Given the different process management environments ranging from initial synthesis to packaging, pharmaceutical and life sciences plants face major challenges to understand the total process. NWA solutions help them to collect data at each step of the production process, analyze it, and then make it visible for good decision support. With high ease-of-use and configurable-off-the-shelf approach, NWA solutions have a minimal impact on IT and a fast time-to-ROI.
NWA delivers the software that turns more data into information, makes it more accessible and delivers role-specific reporting and visualization that is a critical part of FDA CGMP compliance while supporting facility benchmarking and continuous process improvement.
Data Collection and Integration
The typical pharmaceutical plant collects process data with many different systems ranging from in-process control systems to the laboratory. For world-class process management this diverse data must be brought into a unified vision. NWA’s superior data-interchange system aggregates data from all manufacturing and operations databases to deliver a comprehensive process management view. This includes data from:
- Control systems
- Quality Information Systems
- Laboratory Information Management Systems
- Manufacturing Enterprise Systems
.png) Pharmaceutical companies also face the additional challenge of integrating data in multi-vendor environments. Most have some history of acquiring plants which contain many generations of manufacturing systems from multiple vendors.
Pharmaceutical companies also face the additional challenge of integrating data in multi-vendor environments. Most have some history of acquiring plants which contain many generations of manufacturing systems from multiple vendors.
An effective MI solution is database agnostic, independent of manufacturing systems vendors, and reads all data with little or no IT overhead. NWA has extensive working experience with major Historians, MES and LIMS databases. For example, NWA software is incorporated in many commercial LIMS and NWA also collaborates with major control and manufacturing systems vendors.
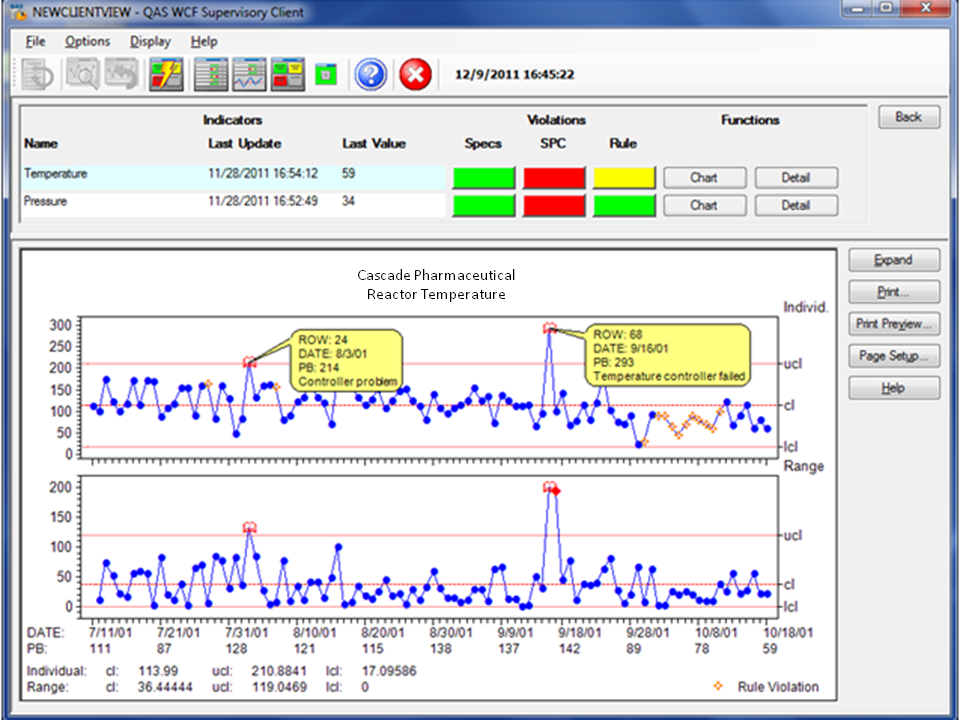 NWA takes data from different environments such as control data from a process historian and test data from the LIMS and aggregates the data into usable process views. NWA analytics software taps into multi-system environments to seamlessly access all relevant data. This provides decision support “standardization” in the absence of systems standardization. Often, NWA dashboards and visualization become the primary user interface that provides a unified quality system view and supports CGMP.
NWA takes data from different environments such as control data from a process historian and test data from the LIMS and aggregates the data into usable process views. NWA analytics software taps into multi-system environments to seamlessly access all relevant data. This provides decision support “standardization” in the absence of systems standardization. Often, NWA dashboards and visualization become the primary user interface that provides a unified quality system view and supports CGMP.
“The next level of productivity gains in manufacturing must be achieved through Manufacturing Intelligence”
Pierfrancesco Manenti, IDC Manufacturing Insights
Analytics and Visualization
Manufacturing Intelligence transforms raw data into actionable information to support pharmaceutical process management and compliance. NWA provides a full suite of industry standard SPC and advanced analytics plus pharmaceutical specific modules such as stability analytics and multivariate SPC.
NWA Stability Analytics™ delivers the statistical analysis, charting, and reporting required for routine product lot stability studies and shelf life prediction under the FDA/ICH guidelines.
NWA MvSPC™ is a fully integrated solution that incorporates multivariate modeling and SPC charting appropriate for many pharmaceutical processes.
Common pharmaceutical manufacturing concerns include low yield and FDA compliance. Analytics such as SPC support good manufacturing decision making. The NWA interface provides full access to all data so users can quickly drill down to specifics and improve yield and compliance.
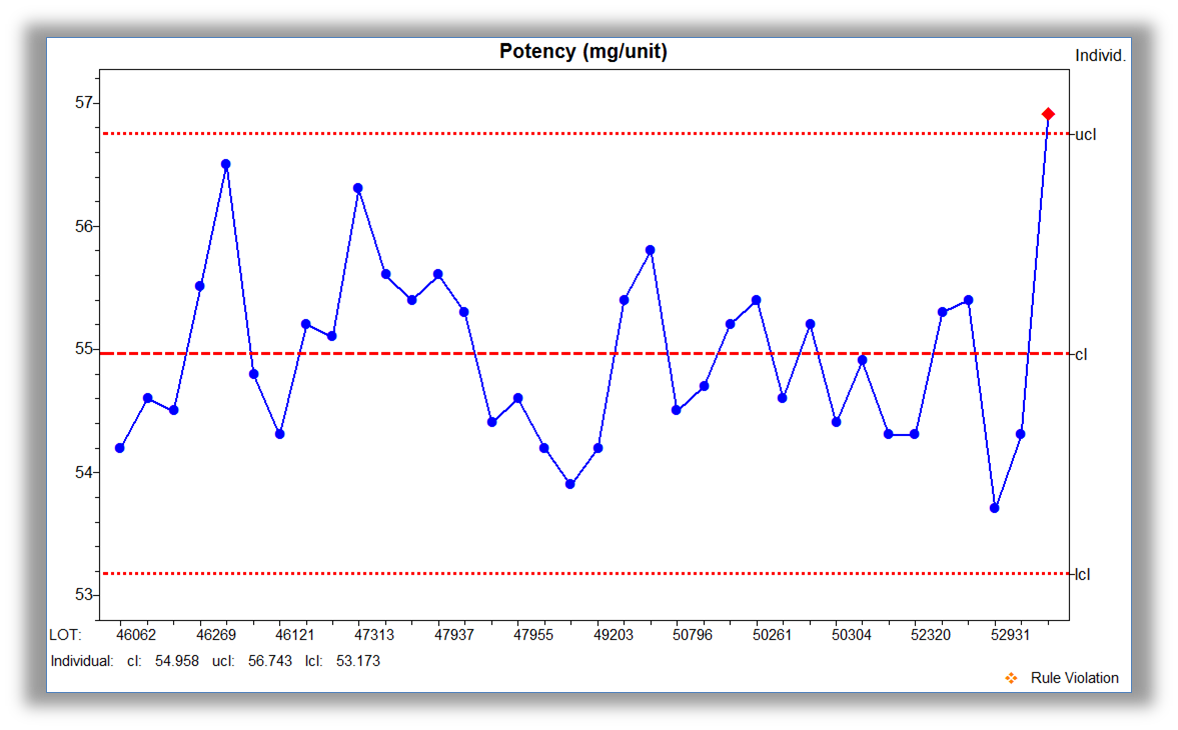 Accessible displays that incorporate analytics give meaning to the data. For over 25 years, NWA has incorporated user feedback to develop visualization that is information rich and easy to interpret with minimal training. Real-time display capabilities include:
Accessible displays that incorporate analytics give meaning to the data. For over 25 years, NWA has incorporated user feedback to develop visualization that is information rich and easy to interpret with minimal training. Real-time display capabilities include:
- Role-based dashboards
- Plant-floor workstations
- Control room monitoring
- Plant-wide visibility
- Corporate oversight
- Supply-chain quality reporting
“Looking for trends once a year is not cGMP anymore.”, James Blackwell
Collaboration
Pharmaceutical process management depends on real-time analytics and alerts to anticipate or catch process issues in a timely fashion. NWA software uses both network and web-based communication to reach all stakeholders within the company and its complete supply chain including suppliers and contract manufacturers. The resulting accessible real-time analytics and visualization proliferates best practices and maintains compliance.
NWA solutions communicate effectively at all three critical levels: across the plant, across the company and across the supply chain.
Across plant – Plant operators and technicians need real-time performance analytics to run the process. Process engineers need the information to support six sigma and continuous process improvement programs. Supervisors and managers need oversight capability and everyone needs the analytics in a role specific report that is optimized for their job and gives them a dynamic understanding of their part of the process.
Across Company – Corporate engineering and management need process and business data analyzed to benchmark plant performance and determine best practices for company plants. Real-time alerts and reporting enable management to properly handle risk issues such as CAPA programs.
Across Supply Chain – Supply chain management depends on timely communication between vendor and customer. In state of the art vendor certification programs suppliers must demonstrate process stability and the capability to produce product to specification. To meet supplier contracts and standards such as ISO 9001:2008, the manufacturer must provide the ongoing quality and process metrics required to fulfill contracts. NWA cover all these circumstances with both client-server and web-based capabilities.
